Cold Chain Protocol - Vaccines
and Biologics
Appendix 5: Cold Chain Failure Response Diagram
What to do if the Temperature of the Refrigerator goes outside of 2-8ºC.
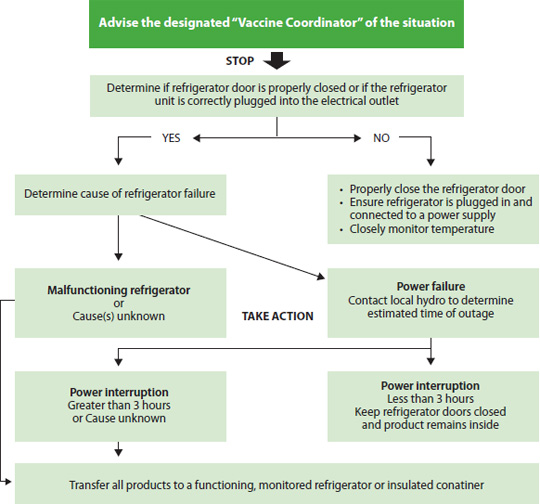
Essential actions:
- Follow the procedures and complete form in the Cold Chain Failure Response Procedure and Form.
- Take inventory of potentially compromised vaccines and biologics.
- Isolate potentially affected product into a separate container (paper bag) marked “do not use” and store in a functioning refrigerator.
- Do not return product to malfunctioning fridge until the unit has been serviced and demonstrated to maintain +2ºC to +8ºC storage temperatures for at least 2 days.
- Do not use potentially compromised vaccine until clear instructions related to their use have been received by the manufacturers or from stability data in the product monographs.
Alert your Vaccine Coordinator that a cold chain failure has occurred regardless of the cause:
Alert your Vaccine Coordinator that a cold chain failure has occurred regardless of the cause:
Regular office hours contact person: __________________________
Phone: _____________________________
After hours contact person: _________________________________
Phone: _____________________________
|
A Cold Chain Failure Response Form must be completed for all cold chain failures.
Consultation with Manitoba Health is required prior to vaccines and biologics being returned.
Cold Chain Failure Response Forms must be faxed to Manitoba Health at 204-948-2040. |
Communicable Disease
Control (CDC)
Public Health
Manitoba Health
4th Floor - 300 Carlton St.
Winnipeg MB R3B 3M9 CANADA
Health Links – Info Santé
204-788-8200 or 1-888-315-9257
|



